Voluntary Recall of NP Thyroid (Generic Natural Desiccated Thyroid)
- Mary Shomon
- May 22, 2020
- 2 min read
Updated: Aug 3, 2020
Acella Pharmaceuticals has issued a voluntary nationwide recall of some lots of NP Thyroid due to "super potency."

Acella Pharmaceuticals is voluntarily recalling 13 lots of 30-mg, 60-mg and 90-mg NP Thyroid® (thyroid tablets, USP) to the consumer level. The products are being recalled because Acella's testing found these lots to be super potent (too strong). The product may have up to 115% of the labeled amount of liothyronine (T3).
Patients being treated with the identified super potent NP Thyroid may experience signs and symptoms of hyperthyroidism including weight loss, heat intolerance, fatigue, muscle weakness, high blood pressure, chest pain, rapid heart rate, diarrhea, anxiety, or heart rhythm disturbances.
If you are taking generic natural desiccated thyroid (NDT), be advised that in the U.S., the generic prescriptions are filled with NP Thyroid.
If you are unsure if your NP Thyroid is from these lots, check with your pharmacist. And if you have any of the recalled NP Thyroid, contact your health care provider.
Don't discontinue use of your NP Thyroid without contacting your healthcare provider for further guidance and/or a replacement prescription.
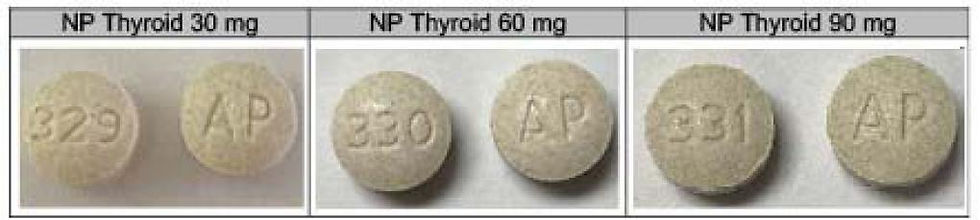
Which NP Thyroid is Affected?
The NP Thyroid being recalled is packed in 100-count bottles. To identify the product, the NDC’s, Product Description, Lot Numbers and Expiration Dates are listed. These lots were distributed nationwide in the USA to Acella’s direct accounts.

Questions or Concerns?
If you have questions about the recall can you email Acella Pharmaceuticals at recall@acellapharma.com or contact Acella Customer Service at 1-800-541-4802, Monday through Thursday from 9:00 am to 5:00 pm ET and Friday from 9:00 am to 12:30 pm ET.
If you had or have an adverse reaction to your NP Thyroid, you should report it to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online
Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178














Comments